This group is defined as the alternating group of degree , i.e., the alternating group on a set of size . In other words, it is the subgroup of symmetric group:S7 comprising the even permutations. Further information: element structure of alternating group:A7.Student worksheet about group 7 elements to be used when teaching GCSE chemistry. In addition, we have premium resources including answer sheets and mark schemes which are aimed at teaching GCSE students group 7 elementsGroup 5 element — A Group 5 element is one in the series of elements in group 5 (IUPAC style) in the periodic table, which consists of vanadium (V), niobium (Nb), tantalum (Ta), and dubnium (Db).All of these elements are classed in Group 5 because their valence… …The main group elements of the periodic table are those elements that belong to the "s" and "p" blocks. Counting the columns or table groups across the table ignoring the transition elements gives 8 element groups which match the filling of the eight spaces for electrons in the ns and np subshells...Elements in group 1A have one valence electron, elements in group 2A have 2 valence electrons, etc. Noble gases have a full outer shell of 8 valence As one proceeds down the group 7A elements, the first ionization energy decreases. this means that the outermost electron is more readily removed...
Group 7 Elements | Teaching Resources
The elements are fluorine ,chlorine,bromine,iodine and astatine. Oxidation number of halogen is -1.All halogen elements have seven electrons in the outer most shell that is valence electrons and Group 17 elements are called halogens because halogen is a Greek word which means 'salt producing'.There are 112 elements although elements 110-112 are as yet unnamed. These 112 elements are organized in the periodic table: The modern chemical symbols were introduced by Berzelius. Rows of elements are called "periods" and columns of elements are called "groups" (1A, 2A 3B etc.).Group 7, numbered by IUPAC nomenclature, is a group of elements in the periodic table. They are manganese (Mn), technetium (Tc), rhenium (Re), and bohrium (Bh). All known elements of group 7 are transition metals.The group number is an identifier used to describe the column of the standard periodic table in which the element appears. Transition elements are those whose atoms have an incomplete d-subshell or whose cations have an incomplete d-subshell. Groups 13-18 termed p-block elements.

Group 7 element | Academic Dictionaries and Encyclopedias
The Group 7 elements are called the halogens. Group 7 elements form salts when they react with metals. The term 'halogen' means 'salt former'.B. transition elements. C. noble gases. 1A (s-block) and 7A(p- block ) are representative elements.Halogens Some of the elements are fluorine, chlorine, bromine, iodine. How are elements arranged in the modern periodic table? What were the contributions of Mendeleev to the periodic table?Reactivity of Group 7 non-metals increases as you go up the group. Out of the 3 halogens, chlorine, bromine and Iodine, chlorine is the most reactive and iodine is the least reactive. Each outer shell contains seven electrons and when group 7 metals react, they will need to gain one outer electron to...4 Comments on Element Infographics - Group 7. This graphic looks at the halogens, found in Group 7 of the Periodic Table. This group consists of the elements fluorine, chlorine, bromine, iodine and astatine - the as yet unnamed artificial element 117, ununpentium, may also be a halogen.
A Group 7 element is one in the series of elements in group 7 (IUPAC taste) in the periodic table, which consists of manganese (Mn), technetium (Tc), rhenium (Re), and bohrium (Bh). All identified elements of group 7 are transition metals.
Like different groups, the participants of this family show patterns in their electron configurations, especially the outermost shells leading to developments in chemical behavior.
Chemistry
H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar Okay Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba * Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn Fr Ra ** Rf Db Sg Bh Hs Mt Ds Rg Cn Uut Uuq Uup Uuh Uus Uuo * La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu ** Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr Group 7 within the periodic desk Z Element No. of electrons/shell 25 manganese 2, 8, 13, 2 43 technetium 2, 8, 18, 13, 2 75 rhenium 2, 8, 18, 32, 13, 2 107 bohrium 2, 8, 18, 32, 32, 13, 2Bohrium has no longer been remoted in pure shape, and its properties have not been conclusively observed; best manganese, technetium, and rhenium have had their homes experimentally confirmed. All three elements are conventional silvery-white transition metals, exhausting, and have prime melting and boiling points.
History
Group 7 incorporates the two naturally occurring transition metals discovered closing: technetium and rhenium. Manganese has been known for millennia. Rhenium used to be discovered when Masataka Ogawa found what he concept used to be component 43 in thorianite, however this used to be dismissed; recent research by way of H. K. Yoshihara counsel that he discovered rhenium as an alternative, a reality no longer realized on the time. Walter Noddack, Otto Berg, and Ida Tacke had been the first to conclusively establish rhenium; it was concept they discovered component Forty three as smartly, but because the experiment could no longer be replicated, it was once brushed aside. Technetium was once officially came upon in December 1936 through Carlo Perrier and Emilio Segré, who found out Technetium-Ninety five and Technetium-97. Bohrium was once discovered in 1981 through a crew led by Peter Armbruster and Gottfried Münzenburg by way of bombarding Bismuth-209 with Chromium-54.
Occurrence
Manganese is the only not unusual Group 7 element. In 2007 11 million metric lots of manganese had been mined. All other elements are either incredibly uncommon on earth (technetium, rhenium) or utterly artificial (bohrium). In contrast to manganese, most effective forty or 50 metric lots of rhenium have been mined. Technetium is most effective present in hint amounts in nature as a manufactured from spontaneous fission; almost all is produced in laboratories. Bohrium is simplest produced in nuclear reactors and has never been isolated in natural form.
Biological OccurrenceOnly manganese has a task in the human frame. It is an essential trace nutrient, with the frame containing roughly 10 milligrams at any given time, being principally in the liver and kidneys. Many enzymes include manganese, making it very important for lifestyles, and is also present in chloroplasts. Technetium, Rhenium, and Bohrium have no known biological roles.
v · d · eGroup 7 elementsManganeseMnAtomic Number: 25Atomic Weight: 54.938045Melting Point: 1519.15 KBoiling Point: 2334 KSpecific mass: 7.Forty four g/cm3Electronegativity: 1.55
TechnetiumTcAtomic Number: 43Atomic Weight: 98Melting Point: 2473.15 KBoiling Point: 5150 KSpecific mass: 11.5 g/cm3Electronegativity: 1.9
RheniumReAtomic Number: 75Atomic Weight: 186.207Melting Point: 3453.15 KBoiling Point: 5869 KSpecific mass: 21.02 g/cm3Electronegativity: 1.9
BohriumBhAtomic Number: 107Atomic Weight: 264Melting Point: ? KBoiling Point: ? KSpecific mass: 37 g/cm3Electronegativity: ?
Solved: INITIALS: 33. The Elements In Group 7A Are Known B... | Chegg.com
What Is The Octet Rule And Why Does It Apply Primarily To Main Group Elements | Course Hero

Solved: Part A For XY4 (X And Y Are Group 7A Elements) Whi... | Chegg.com

Based On Your Answer In Part B, Arrange The Five Elements In Order Of Their Reactivity. Drag The Most - Brainly.com

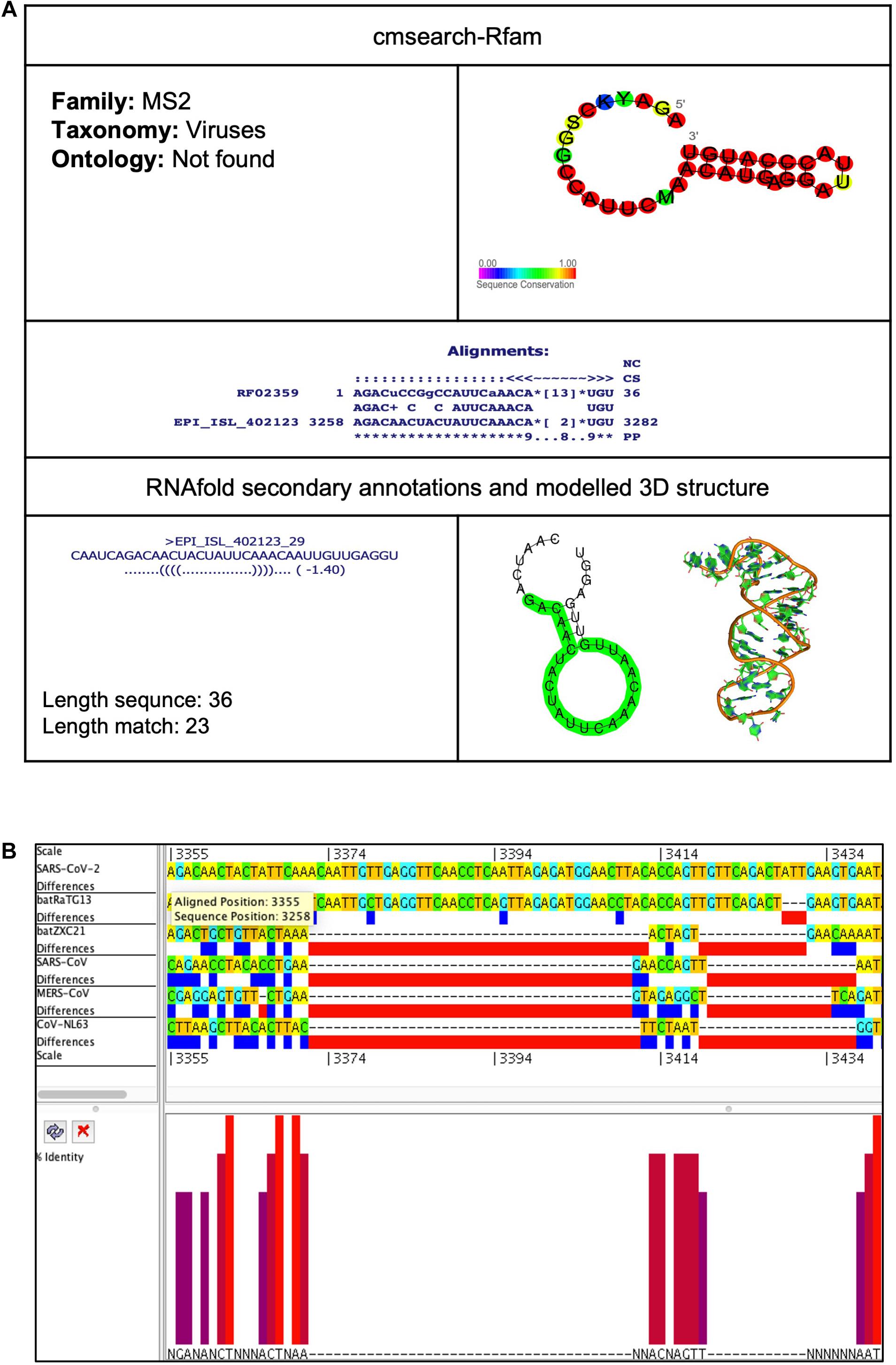
Frontiers | A Comprehensive Analysis Of Cis-Acting RNA Elements In The SARS-CoV-2 Genome By A Bioinformatics Approach | Genetics
Group 7A Elements Cubes By Science Worksheets | Teachers Pay Teachers

Group 7A Elements Cubes By Science Worksheets | Teachers Pay Teachers

Properties & Uses Of Compounds Of Group 2 Elements - Video & Lesson Transcript | Study.com

Oxidation Numbers
Chem 2 AP HW 8

Electron Configuration And The Periodic Table Section 2 Review | Electron Configuration, Geometry Worksheets, Science Web

DATE 2.e – Lewis Dot Structures
Name: Date: Period: Independent Practice: Periodic Trends [Atomic

The Parts Of The Periodic Table

What Are The Group 1A And Group 7A Elements Examples Of? My Last Wuestion - Brainly.com

7a Images, Stock Photos & Vectors | Shutterstock

Chemistry: The Central Science, Chapter 6, Section 9

Binary Ionic Bonding Practice | Interactive Worksheet By Courtney Baines | Wizer.me
Solved: 5. In The Periodic Table, The Element Hydrogen Is | Chegg.com
Combinatorial Recognition Of Clustered RNA Elements By The Multidomain RNA-binding Protein IMP3 | Nature Communications

Periodic Table 8.5C: Periodic Table Re-templated JPh 7/31 - Ppt Download







Tidak ada komentar:
Posting Komentar